Descubra os dispositivos essenciais de teste de integridade de filtros para manter a adequação e integridade dos filtros de membrana no setor.
Os testes de integridade são testes não destrutivos e, como tais, podem ser realizados antes da filtração e depois da filtração, sem afetar o próprio filtro. Ao mesmo tempo, eles fornecem a confiança de que o filtro está intacto e entregando o desempenho esperado e as características de retenção.
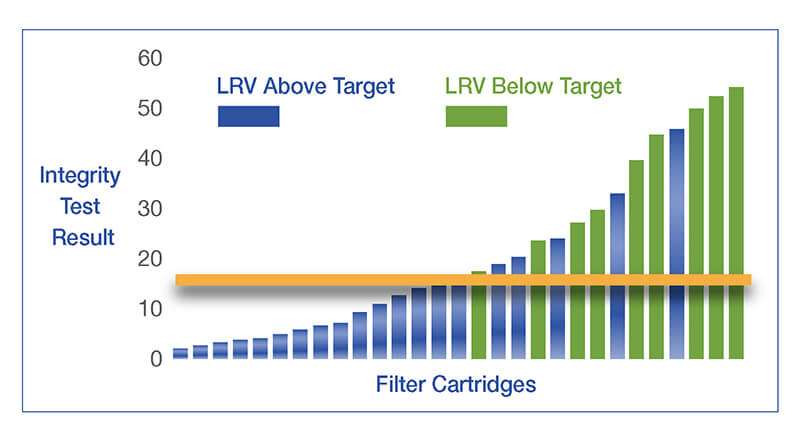
Teste de integridade do filtro durante a validação, relaciona-se à remoção microbiana dos filtros finais com base nas membranas usadas durante o desenvolvimento do produto. Para esse fim:
- Os filtros são testados com vários valores de integridade sob condições específicas e difíceis com micróbios.
- Os resultados do teste de desafio microbiano são plotados contra os valores do teste de integridade para comparação.
- Com base no nível de remoção microbiana desejado — grau de esterilização ou um valor de redução de registro (LRV) específico — um limite de teste aceitável para o teste de integridade é estabelecido (por exemplo, ml/min ou queda de pressão em mbar)
Valores do teste de integridade e desafio microbiológico
Um teste de integridade correlacionado pode confirmar que os filtros estão íntegros e adequados para o serviço contínuo e fornecerá os seguintes benefícios:
- Garante que os filtros estejam na classificação correta, instalados corretamente e livres de danos.
- Os testes de integridade podem ser realizados em uma carcaça do filtro vazia com válvulas a montante e a jusante fechadas para verificar se todos os selos de o-ring estão intactos.
- Confirma que o filtro atende as especificações do fabricante.
- É essencial para a validação do processo, os arquivos de registro de lote e monitoramento e pode ser útil durante a revisão da documentação do processo.
- Finalmente, ele permite uma identificação mais rápida da contaminação.
- Se o filtro passar no teste de integridade e houver contaminação a jusante do filtro, as seguintes causas raízes são as mais prováveis:
- Porta de coleta de amostra contaminada
- Cabeças de alimentação contaminadas
- Lacres contaminados
- Garrafas contaminadas
Soluções de teste de integridade de filtros da Pall oferecendo confiança
100% dos filtros Pall são testados quanto à integridade durante a fabricação antes do lançamento. Um limite de teste de integridade de produção é específico para o método de teste de integridade do local de fabricação e os resultados de validação. Além disso, há um limite para o teste de integridade do cliente adequado para usar com os dispositivos de teste de integridade Palltronic®, fornecido pela Pall para uso pelo cliente(por exemplo, Dispositivo de teste de integridade Palltronic Compact Touch).
Pall Compact Touch (dispositivo para teste de integridade)
Testes de integridade recomendados para confirmar a integridade da redução microbiana e filtros de membrana com grau de esterilização
Dois testes de integridade são recomendados para confirmar a integridade da redução microbiana e os filtros de membrana com grau de esterilização: Fluxo difusivo e Queda de pressão. O valor do fluxo direto está vinculado aos resultados do teste de desafio bacteriano. O valor de queda de pressão é derivado do valor do fluxo direto e do volume a montante da carcaça.
Esses testes estão correlacionados ao nível definido da remoção microbiana ou à redução do nível durante a validação inicial do filtro. Como testes não destrutivos, eles podem ser usados depois da higienização e ao final de um lote de produção para mostrar que o conjunto do filtro está íntegro. Esses testes são baseados nos mesmos princípios que regem o fluxo de gás através de um poro umedecido.
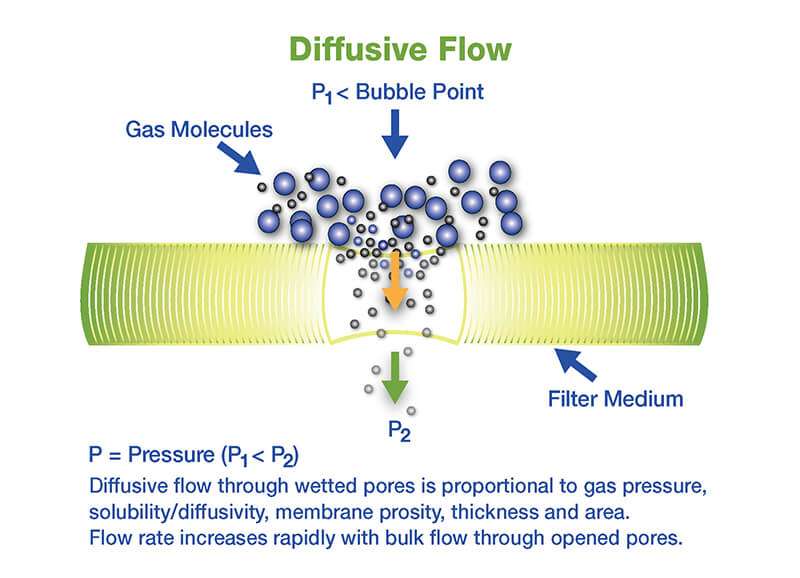
Teste de fluxo difusivo
- A pressão dentro do volume a jusante da carcaça do filtro é mantida no filtro específico, pressão do teste de fluxo difusivo.
- O fluxo de gás total, formado pelo fluxo difusivo através de poros umedecidos e o fluxo a granel através dos poros abertos ou defeitos através do filtro são medidos pelo dispositivo de teste a jusante ou a montante, que mede o fluxo difusivo mede o fluxo exigido para manter a pressão de teste.
Teste de queda de pressão (também conhecido como retenção de pressão)
- A pressão dentro do volume a montante da carcaça do filtro é estabilizada no filtro e carcaça específicos, a pressão do teste de queda de pressão.
- O dispositivo de teste a montante mede o fluxo total de gás através do filtro.
- A queda de pressão mede a redução da pressão.
Teste de intrusão de água (WIT)
- O fluxo de água é medido através de um filtro hidrofóbico e correlacionado à retenção microbiana.
O teste de retenção bacteriana é o teste de integridade mais sensível para a capacidade de esterilização e redução microbiana. O teste de integridade do filtro pode ser destrutivo e pode contaminar o filtro com bactérias do teste, tornando-o impróprio para uso. Se devidamente correlacionados, testes não destrutivos podem indicar o quão bem o filtro retém as bactérias mais difíceis.
Considerações práticas ao realizar testes de integridade
| Seleção do fluido de teste de integridade | Fluidos de teste de “referência” padrão
Água: filtros hidrofílicos e hidrofóbicos
Álcool/água: filtros hidrofóbicos
Fluidos do processo (valores derivados)
|
| Seleção do gás para pasteurização | Gases de testes aceitáveis:
Gases de teste inaceitáveis:
|
| Temperatura | A temperatura deve permanecer dentro de 5 °C durante um teste de integridade. Mudanças significativas de temperatura podem alterar a difusão de gás do fluido. A faixa de temperatura recomendada para o teste de integridade é 20 °C ± 5 °C (68 °F ± 9 °F). Uma mudança de temperatura de 1 °C causará uma alteração de volume aproximada de 0,3% devido ao seu impacto crítico na pressão do gás. |
| Volume a montante | O volume a montante é uma consideração essencial para os testes de queda de pressão. Nas aplicações onde os filtros são regenerados de modo eficiente, sua vida útil em serviço será ditada pela integridade da sua estrutura. |
Palltronic® Compact Touch
Saiba mais sobre a próxima geração de dispositivos de teste de integridade da queda de pressão, projetados especificamente para os fabricantes de alimentos e bebidas.